Congratulations to Troy and KaReisha for being recognized with Travel Awards to the 2022  Autumn Immunology Conference sponsored by the National Multiple Sclerosis Society and the John Wallace Diversity Award, respectively!
Autumn Immunology Conference sponsored by the National Multiple Sclerosis Society and the John Wallace Diversity Award, respectively!
Author: selutz
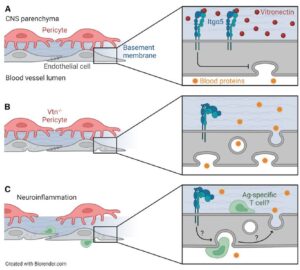
Trevino et al., 2022 published in Neuron
Perspective published in Neuron on the role of vitronectin in suppressing BBB leakage
New award-winning lab member!
Welcome Ali Almousawi, our newest lab member. Ali is an undergraduate major in Neuroscience with a minor in Life Science Visualization, with future plans to study neuroimmunology in graduate school. Ali has been selected for the Chancellor’s Undergraduate Research Award.
Congratulations Troy
Congratulations to Troy Trevino for being awarded a T32 Predoctoral Fellowship in the Vascular Biology, Signaling and Therapeutics (VBST) training program (T32 HL144459).
Troy was also selected for a platform presentation at the Cold Spring Harbor Labs Brain Barriers meeting (April 2021).
Undergraduate Fellowship Award!
Congratulations to Quinton Crisman for receiving a prestigious LASURI Undergraduate Research Initiative fellowship! This fellowship will fund Quinton for lab research in both semesters of the 2021-2022 academic year and allow him to present his work at a conference. Quinton’s project is “Neurovascular inflammation in a novel mouse model of COVID-19”.
Undergraduate Fellowship Award
Congratulations Vidya Babu for being awarded a LASURI fellowship to support her research in the lab during Fall semester 2020.
Invited seminars
Dr. Lutz is invited to present her laboratory’s research at University of California San Diego as a CCTS Visiting Scholar (Spring 2021), at Rush University Department of Microbiology (Spring 2021), and special seminars at UIC Microbiology & Immunology (Fall 2020) and Grand Rounds for UIC Neurology (January 2021, zoom link TBA)
Congratulations Troy
Troy did a stellar job on his qualifying exam and advanced to candidacy for the doctoral degree in the UIC GEMS PhD program. Troy also won a presentation award for his e-poster at the Center for CardioVascular Research Day in September 2020.
Welcome Quinton Crisman
Quinton Crisman joined the lab as an undergraduate research fellow. His main project is to investigate how COVID-19 alters the blood-brain barrier. Outside the lab, Quinton’s interests include rock climbing and performing improv comedy at Second City.
Best Poster Award
Troy Trevino tied for first place in the best poster award competition at the Center for CardioVascular Research Conference, for his work on chemokines and BBB permeability.