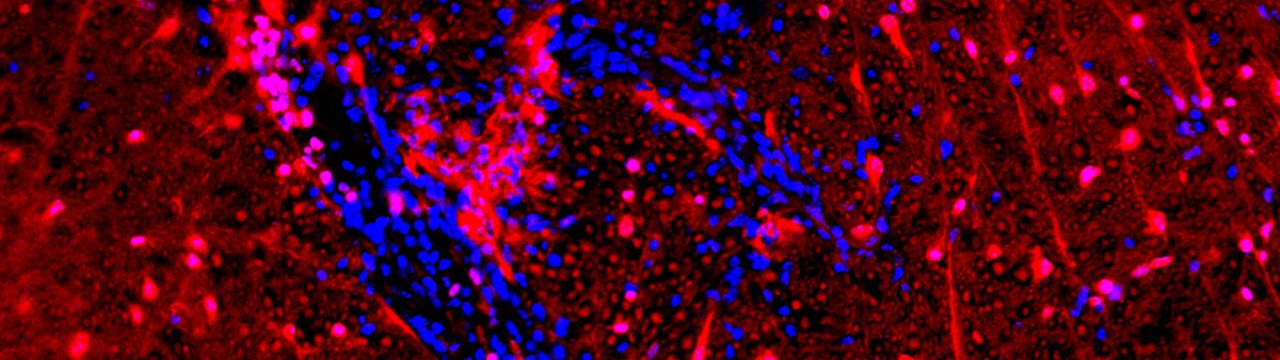
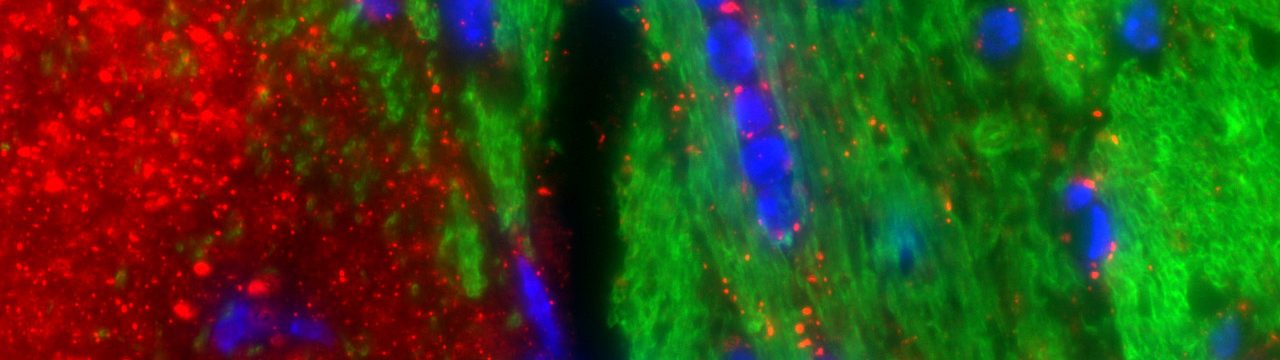
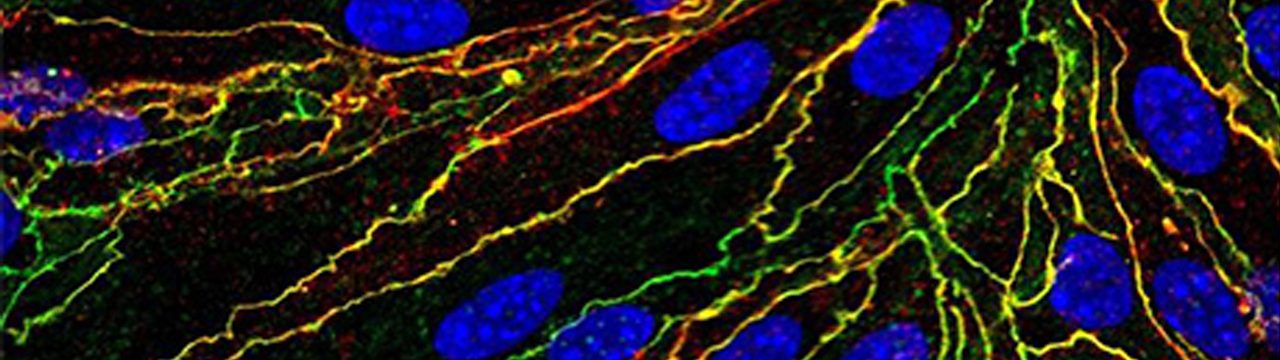
My co-first-author paper describing a novel role for the Wnt/beta-catenin signaling pathway in protection of blood-brain barrier endothelial cells has been published in Proceedings of the National Academy of Sciences on January 30, 2017 (Lengfeld, Lutz, et. al.). Endothelial cells (ECs) in the CNS form a unique blood–brain barrier (BBB) that is broken down in multiple sclerosis (MS). New therapies are sorely needed to restore BBB function in this disease. The wingless integrated MMTV (Wnt)/β-catenin pathway, which is essential for barrier formation, is activated in CNS ECs in MS and the animal model experimental autoimmune encephalomyelitis. When this pathway is inhibited in ECs before disease onset, mice develop more severe disease, with more immune cells entering the CNS owing to increased levels of proteins that promote interactions of immune cells with blood vessels and caveolar transport across vessels. Our findings suggest that pharmacologic enhancement of Wnt signaling may be helpful to limit BBB damage and CNS immune cell infiltration in MS. This work was conducted at the University of California Irvine and at Columbia University Medical Center.
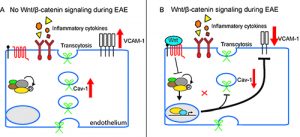
Figure 1. Endothelial cell Wnt/b-catenin activation protects the neurovascular unit in the EAE model of multiple sclerosis. A) In the absence of Wnt signaling, neuroinflammation is exacerbated by elevated caveolar transcytosis and vascular cell adhesion molecule (VCAM-1) expression. B) Wnt/β-catenin mediated transcription suppresses caveolar transcytosis and VCAM-1 expression, markedly reducing T cell infiltration into the CNS.
View the abstract at: http://www.pnas.org/content/early/2017/01/26/1609905114.abstract